Blogs
Bot Image On Trial Site News
Trial Site News features the latest coverage of clinical research. Read the article here:
ProstatID Passes FDA 510(k) Administrative Review
The Bot Image team has been hard at work trying to obtain FDA clearance. We’re excited to announce that we have passed the first major milestone on July 21, 2020.
Successful Beta Test
We’re proud to announce that in partnership with our beta testing site, we processed our first test case using the cloud-based architecture on 6/18/2020.
A Conversation With Our CEO Dr. Randall Jones, PhD, MBA
We recently sat down with our CEO in his library to discuss the mission, vision, and values of Bot Image. We discussed ProstatID and the…
10 Questions With Our Founder
We recently sat down with our founder to ask 10 questions about the company, the industry, and what are the options men have when facing…
Saving Lives, Improving Prostate Cancer Detection with Artificial Intelligence
A prostate cancer diagnosis can be devastating news for the one in seven men who develop the disease in their lifetimes. Annually, 30,000…
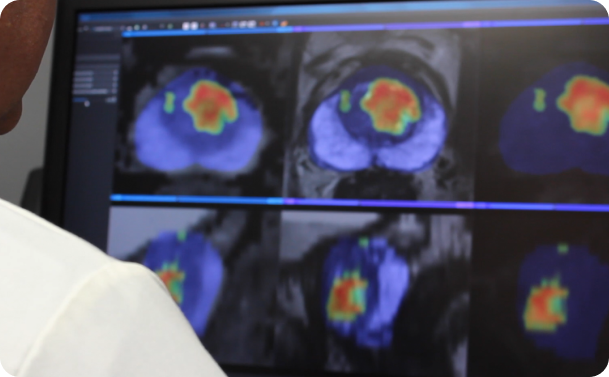
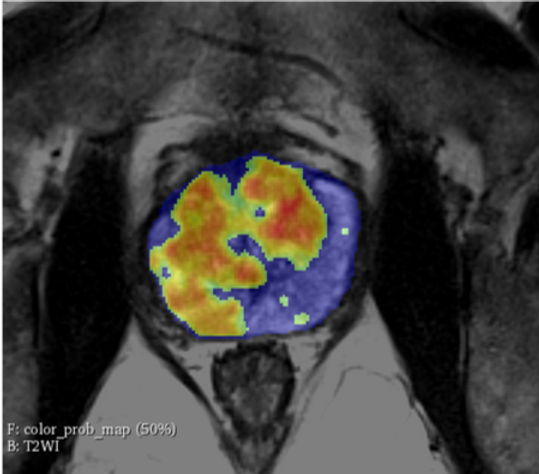
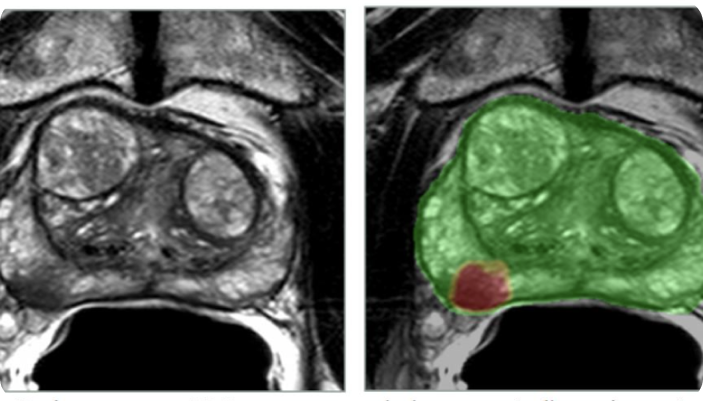
Bot Image highlights performance of ProstatID in study
AI medical device company Bot Image is highlighting the performance of its ProstatID in a study evaluating its ability to detect, diagnose, and screen for prostate cancer.
The Use Of Prostatid In Cancer Detection, Diagnosis And Screening
Overview: ProstatIDTM is North America’s first FDA-Cleared Prostate Cancer SCREENING, Detection and Diagnostic AI software. With 94% AUROC (Area under
An Overview of Prostate Cancer Detection Standard of Care and The Role of MRI for Early Detection of Prostate Cancer
Randall W. Jones, D.Eng. (PhD, MBA) EARLY DETECTION through Screening Population-Based Screening Studies Screening for Prostate Cancer (PCa) continues to